Osteoporosis
The silent disease affecting 200 million women worldwide
Home » Osteoporosis
Overview
Osteoporosis is a disease characterized by low bone mass and structural deterioration of bone tissue, which leads to greater fragility of bones and an increase in fracture risk.
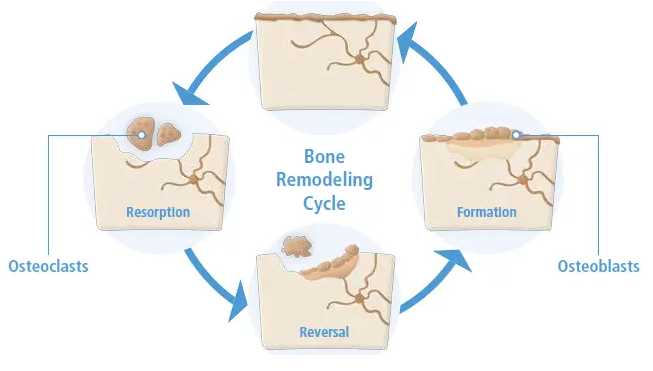
Osteoclast and Osteoblast Imbalance: A Pathway to Osteoporosis
The bone remodeling cycle can be separated into two distinct processes: (i) bone resorption, where cells called osteoclasts function in the resorption of mineralized tissue and (ii) bone formation, where cells called osteoblasts are responsible for bone matrix synthesis and subsequent mineralization of the bone. In healthy individuals, bone resorption is matched by new bone formation.
Osteoporosis develops as the balance between bone resorption by osteoclasts and bone formation by osteoblasts is not maintained, and not enough bone tissue is formed, leading to frail and fracture-prone bones.
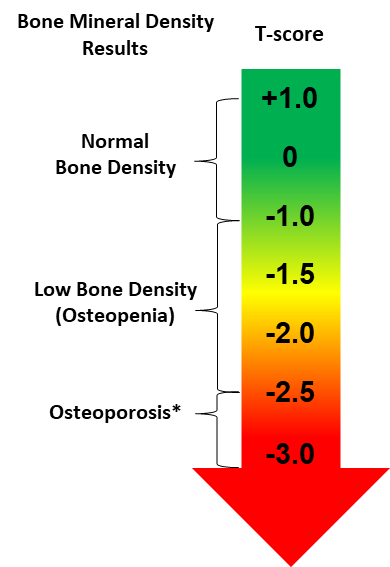
Osteoporosis is Diagnosed and Managed via Bone Mineral Density (BMD) T- Score
- Standard bone density test is used to measure bone mineral content and density
- Non-invasive X-rays, dual-energy X-ray absorptiometry (DXA) scan to determine bone density of the hip, femur or spine
- T-Score stages severity of low bone mass and osteoporosis
Market Need Driven by Aging Population
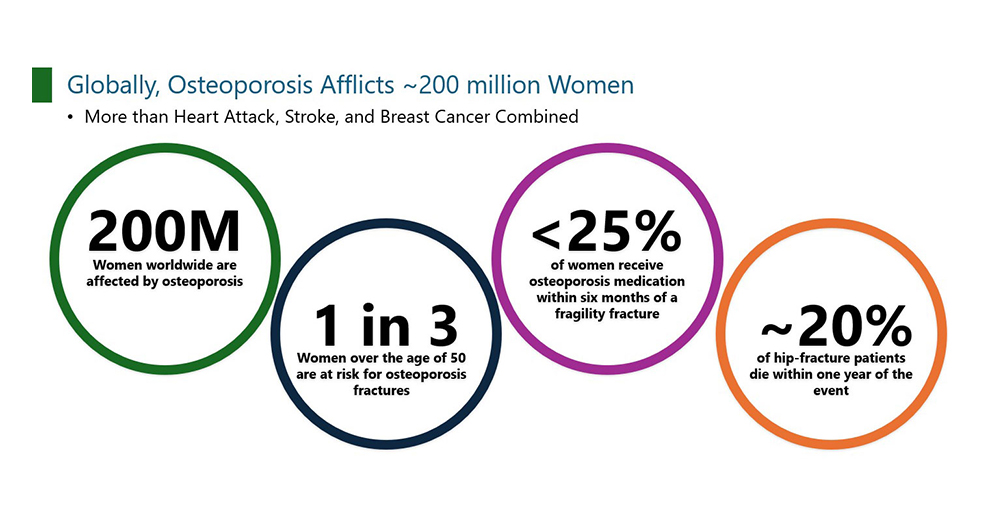
Osteoporosis is a global public health problem currently affecting more than 200 million people worldwide. Of the estimated 15 million patients diagnosed with osteoporosis in the United States, 13 million are women. According to recent statistics from the International Osteoporosis Foundation, worldwide, 1 in 3 women over the age of 50 years and 1 in 5 men will experience osteoporotic fractures in their lifetime.
Osteoporosis results in a decreased quality of life, increased disability-adjusted life span, and a tremendous financial burden to health insurance system. In the United States, for women aged 55 years and older, the hospitalization burden of osteoporotic fractures and population facility-related hospital cost is greater than that of heart attack, stroke, or breast cancer.
Need for New, Differentiated
Treatments for Osteoporosis
It is estimated that approximately 3.2 million patients are treated with osteoporosis medication in the U.S. alone. However, less than 10% of osteoporosis patients use current anabolic (bone building) drugs. Despite the validated mechanism of action of these treatments, patients are deterred by their high cost and injectable mode of administration. Instead, the most prescribed class of drugs for osteoporosis are bisphosphonates, which come in more convenient oral administration regimens. There exists a treatment gap due to poor patient acceptance of injectible anabolic drugs. There are currently no FDA-approved oral anabolic treatments for osteoporosis.
EB613:
First Oral Osteoanabolic Tablet Addresses Current Treatment Chasm
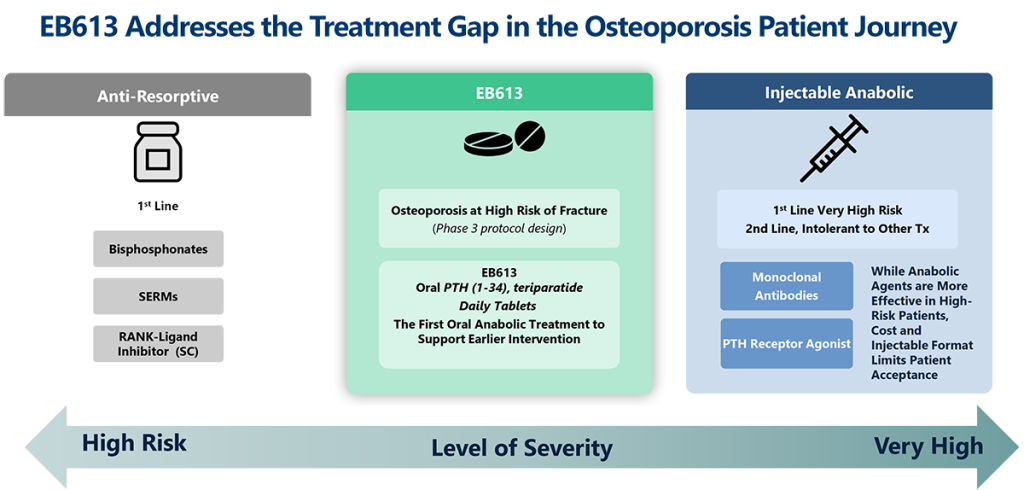
Dual anabolic and anti-resorptive MOA in the convenience of a daily tablet
Unique and vastly expanded patient population per Phase 3 design
All caregivers Endocrinologists, PCP, rheumatologist, Gynecologists buy-in according to vast market research
Cost advantage versus injectables
Enabling patients to actively manage their disease and deter decrease fracture risk

